Abstract
Background TKIs have improved outcomes of patients with CML. However, therapeutic resistance (BCR-ABL1 mutation-dependent or -independent) remains a clinical challenge. The BCR-ABL1 T315I mutation ("gatekeeper") is insensitive to first- and second-generation TKIs, and compound mutations complicate management with all TKIs (including the third-generation TKI ponatinib). Olverembatinib is an orally active BCR-ABL1 TKI with antitumor activities and was found in clinical trials to have a preliminary favorable safety profile for the treatment of patients with chronic phase or accelerated phase CML (CML-CP/CML-AP) that failed prior TKI therapy.
Methods This first-in-human phase 1 trial evaluated the safety and efficacy of olverembatinib in adults with CML-CP or CML-AP resistant or intolerant to first- or second-generation TKIs. Patients with severe cardiovascular diseases, hypertension, and pulmonary arterial hypertension were excluded. Olverembatinib was orally administered once every other day in 28-day cycles in 11 dose cohorts ranging from 1 to 60 mg. This study reports data on patients with long-term follow-up.
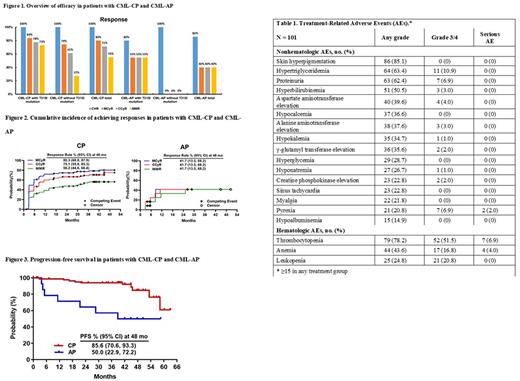
Results From October 26, 2016, to April 30, 2022 (data cutoff date), 101 patients with CML-CP (n = 86) and CML-AP (n = 15) were enrolled and treated with olverembatinib. Seventy-one (70.3%) patients were male, with a median (range) age of 40 (20-64) years and a median (range) interval from diagnosis to initial olverembatinib treatment of 6.0 (0.3-15.2) years. Median treatment duration was 44.7 (1.2-63.1) months. A total of 84 (83.2%) patients received ≥ 2 lines of TKI therapy, and 63 (62.4%) had disease harboring the T315I mutation. At baseline, compound mutations were detected in 12 (11.9%) patients, of whom 8 (66.7%) harbored the BCR-ABL1T315Imutation. A total of 20 (19.9%) patients had 2 (n = 13) or ≥ 3 (n = 7) mutations. As of the data cutoff date, 72 (71.3%) patients continued treatment and 28 (21 CML-CP and 7 CML-AP) discontinued because of disease progression, intolerance, or other reasons. Cumulative median (range) drug exposure was 20,175 (660-34,395) mg. Of 101 patients, 79 (78.2%) were treated > 3 years, 21 (20.8%) > 4 years, and 3 (3.0%) > 5 years. Of evaluable patients with CML-CP, 100% experienced complete hematologic responses (CHR), 80% major cytogenetic responses (MCyR), 71.3% complete cytogenetic responses (CCyR), and 55.3% major molecular responses (MMR). Of the CML-AP patients, 85.7% had CHR and 40% each for MCyR, CCyR, and MMR. Of evaluable patients with the T315I mutations, 100% of those with CML-CP experienced CHR, 83.7% MCyR, and 73.1% MMR and 80.0% with CML-AP had CHR and 54.5% each for MCyR and MMR. PFS at 48 months was 85.6% (95% CI: 70.6%-93.3%) in patients with CML-CP and 50.0% (95% CI: 22.9%-72.2%) in patients with CML-AP. In 12 patients with compound mutations, next-generation sequencing confirmed that 7 (58.0%) experienced MMR and 3 (25.0%) MR.4.5 At last follow-up, 3 patients had progressed to CML-AP or CML-BP and died, and 7 remained on olverembatinib. One patient had a MMR and 2 each CHR, CCyR, or MR4.5. Most treatment-related adverse events (TRAEs) were grade 1-2. The most frequent nonhematologic AE was, primarily, grade 1-2 skin hyperpigmentation (85.1%). Grade ≥ 3 nonhematologic AEs included hypertriglyceridemia (10.9%), pyrexia (6.9%), and proteinuria (6.9%). The common hematologic TRAE was thrombocytopenia, which was observed in 79 (78.2%) patients, including 52 (51.5% of total population) with grade ≥ 3 and 7 (6.9%) with serious AEs. Leukopenia was grade ≥ 3 in 21 (20.8%) patients but not serious, while anemia was grade ≥ 3 in 17 (16.8%) and serious in 4 (4.0%).
Conclusions The 5-year follow-up results of this first-in-human trial show durable responses and good tolerance of olverembatinib in heavily pretreated patients with CML. Olverembatinib also demonstrated meaningful potency in patients with CML with compound mutations. Internal study identifier: HQP1351-SJ0002.
Disclosures
Chen:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Niu:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Men:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Wang:Ascentage Pharma: Current Employment. Yang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Leadership, Patents & Royalties. Zhai:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Leadership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal